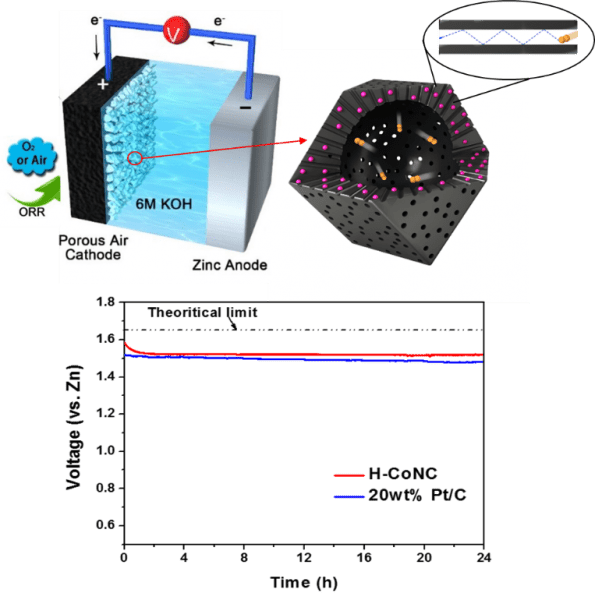
Schematics of the Zn-air battery and O2 confinement effect in the hollow carbon catalyst. Open circuit potential vs. time curves for the batteries based on hollow carbon and commercial 20 wt.% Pt/C.
Metal air batteries are promising power sources for electric vehicle applications, because of their high theoretical energy density (e.g. 1084 W h kg-1 for Zn-air). The practical battery performance is largely constrained by the low solubility of oxygen in the liquid electrolyte, and the sluggish oxygen reduction reaction (ORR) that takes place at the liquid-gas-solid interface in the air electrode.
To address those challenges, Dr. Xiaokai Song and Prof. Jianhua Sun from Jiangsu University of Technology, and Prof. Xiaopeng Li from Shanghai Advanced Research Institute, Chinese Academy of Sciences (SARI-CAS), a hollow cobalt and nitrogen co-doped carbon nanopolyhedra catalyst (H-CoNC), which effectively trap O2 molecules available in the electrolyte is fabricated.
The O2 molecules confined in the porous shell of H-CoNC exhibited longer residence time and increased collision frequency with the electrified inner wall. Moreover, any unreacted O2 molecules passing through the porous carbon shell is trapped in the inner macro-cavity of H-CoNC, which may induce O2 molecules in the inner void to be accumulated with respect to the external electrolyte. As a result, the H-CoNC showed a significant improvement in the ORR kinetics, as well as enhanced ORR selectivity/stability, and resistance to methanol poisoning. The H-CoNC based Zn-air battery yielded an impressive record-high open circuit potential (1.59 V vs. Zn, stabilized at 1.52 V), high power density (331.0 mW cm–2), and promising rate performance.
The preparation of the H-CoNC is based on a novel in situ template evaporation strategy. The ZnO nanosphere firstly acted as the template for the growth of bimetallic metal organic framework (MOF) precursor. Then, the ZnO core was in situ reduced and evaporated during one-step pyrolysis. Compared with conventional approaches, the excess Zn evaporation not only created the inner cavity, but also provided other structure merits of H-CoNC, such as preserving the high nitrogen content, enlarging the hierarchical pores and subsequently, homogeneous ORR active Co-Nx distribution.
This work was published in Small, and was featured as the Cover of Issue 23, 2017.
 Kindly contributed by the Authors of the original manuscript.
Kindly contributed by the Authors of the original manuscript.